ART Engineers Elaborate on Innovations & Operational Issues Affecting Hydrotreater Performance
Grace Davison and Advanced Refining Technologies (ART) FCC/Hydrotreating Symposiums have provided refiners with a forum for in-depth discussions of important developments and operational issues affecting FCCUs and hydrotreaters.
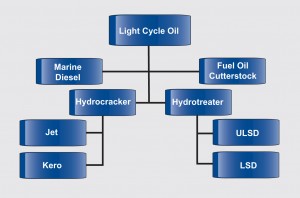
The need to provide technically advanced solutions and cost effective options are why these organizations have invested in advancing refining catalyst technology and related technical support. These include FCC catalysts, as well as hydroprocessing catalyst systems developed through ART (Advanced Refining Technologies), a joint venture with Chevron Products Company. Through ART, a comprehensive line of catalysts for combined conversion processes including distillate hydrotreating, fixed bed and ebullating bed resid hydrprocessing applications as illustrated in the accompanying figure.
Many of today’s refineries require unit-specific technical solutions that in many cases directly affect hydrotreating profitability and efficiency. Against this backdrop of operational concerns, key process variables affecting hydrotreating operations, include:
- Weighted average bed temperature (WABT)
- Liquid hourly space velocity (LHSV)
- Hydrogen
- H2S effects
- Feed effects
- Determination of H2 consumption and heat release
- Pressure drop.
Importance of Temperature
Achievement of a higher WABT will result in higher removal of sulfur and nitrogen via hydrodesulfurization (HDS) and hydrodenitrification (HDN) conversion, respectively. Higher WABT will also result in higher aromatic or polynuclear aromatic (PNA) saturation until the thermodynamic limit is reached.
Hydrotreating reactions are exothermic, making certain temperatures important to specific reactions, which is why a WABT for each reactor is calculated as follows:
WABT = xi (TINi + ⅔[ΔTi]) – EIT
Where:
WABT= weighted average bed temperature
xi = fraction of catalyst in a given bed (i) or reactor
EIT = equivalent isothermal temperature.
WABT determines the level of sulfur and nitrogen removal for HDS and HDN reactions, while the outlet temperature determines the conversion for aromatic or PNA saturation. The inlet temperature (TIN) is the control variable, but is not very meaningful when discussing catalyst activity due to the exothermic nature of hydroprocessing reactions, while ΔT is an indication of a given feed’s reactivity (at given conditions) on a given catalyst. However, this is not a good way to compare catalysts, unless the feed and conditions are identical.
As reactor WABT increases, product sulfur decreases. For example, when processing a 1.1 wt% sulfur LCO blend at 1.0 LHSV (where LHSV = volumetric charge rate/catalyst volume) at1000 psig and 2100 scfb H2/Oil, product sulfur drops to approximately 50 ppm at WABT of 629°F, and drops down to approximately 5 ppm sulfur at a WABT of 670°F.
As a rough approximation, 1/LHSV is approximately equal to the residence time. Higher feed rates correspond to higher LHSVs and higher product production rates, but require higher WABTs to maintain product specifications. As residence time is reduced (i.e., higher LHSV as per 1/LHSV approximation), the required temperature required to maintain the same product sulfur is also increased.
However, higher WABTs come with costs. This is because higher temperatures reduce run length (TEOR – TSOR) and increase coking (fouling). The net result is that higher LHSVs result in shorter cycle lengths. As a “rule of thumb,” fouling rate is proportional to (LHSV) 2 – 3.
In estimating EOR, determination of end-of-run (EOR) is highly dependent on the process feedstock. For example, for naphtha hydrotreating, mercaptan recombination, catalyst poisoning (e.g., silicon [Si], arsenic [As], etc.) and pressure drop determine EOR. Catalyst poisoning (e.g., nickel [Ni], vanadium [V], Iron [Fe] and As), yield losses, furnace and metallurgical limits determine EOR when hydrotreating VGO.
Importance of hydrogen partial pressure
Hydrogen partial pressure (H2PP) plays a significant role in hydroprocessing operations. As a rule-of-thumb, catalyst deactivation rate is proportional to the reciprocal of the hydrogen partial pressure factored by an exponential ranging from 2 to 3:
Catalyst deactivation rate α (1/H2PP)2-3
The H2PP has a large impact on fouling rate, aromatics and PNA saturation (i.e., large effect on product properties related to aromatics like product density, diesel, color and cetane). H2PP also has a significant impact on required WABT for HDN and a moderate impact on required WABT for HDS (but large in ULSD). H2PP is determined by:
- Reactor design pressure
- Make-up hydrogen purity
- Recycle gas rate and purity (important for H2PP at the reactor outlet)
- Bleed or purge rate
- Hydrogen consumption
- Degree of feed vaporization.
The effect of H2PP on HDS and HDN in VGO service was discussed in detail and graphically illustrated by ART experts, as well as H2PP on HDS in ULSD service. A 20°F improvement in HDS is observed with increased H2PP when processing 19.6 API gravity and 1.81 wt% sulfur feed at 1.0 LHSV and 2500 scfb H2/oil reactor conditions. However, a loss of 20°F could occur if the unit operation is allowed to change with less or lower quality hydrogen or lower separator pressures. In addition, a 70°F improvement in HDN is observed with increased H2PP. However, a loss of 70°F could occur if the unit operation is allowed to change with less or lower quality hydrogen or lower separator pressures. It is important to note that for improved HDS in ULSD service, the required WABT delta declines with increasing H2PP. For example, with a 33.0 °API feedstock and 1.66 wt% sulfur, the required WABT delta approaches 0 at 800 psig H2PP (0.75 LHSV, 3000 scfb H2/oil).
The effects of increasing gas-to-oil ratio (scfb H2/oil) raises H2PP, reduces reactor outlet H2S concentration, increases unit activity and stability and increases reactor pressure drop (ΔP). The following gas rate definitions include H2 availability:
Total treat gas rate = Make-up gas rate + Recycle gas rate – ½ Quench gas rate.
Hydrogen gas rate = (Recycle gas – ½ Quench) × (mol% H2 + make-up gas × mol% H2).
H2/Oil = Hydrogen gas rate/Barrels of oil (all in same units, hour or day).
H2 availability = (H2/Oil) / (Chemical H2 Consumption).
Minimum H2 availability recommendations include:
- ≥ 3.0 for straight-run
- ≥ 4.0 for cracked stocks
- ≥ 5.0 for straight-run (ULSD hydrotreating)
- ≥ 6.0 for cracked stocks (ULSD hydrotreating).
Impact of cracked stocks
Cracked stocks are typically more difficult to hydrotreat and require higher WABT compared to straight-run (SR) material at similar product targets. Coker distillates and gas oils have higher sulfur, nitrogen and olefins (Bromine number) with a higher heat release from olefin saturation and higher feed sulfur. Their aromatic content is similar to SR. However, FCC LCO has a higher total aromatics and PNA content (i.e., much lower °API vs.SR or coker) and more olefins that SR, but less than coker material. FCC LCO also has a higher heat release due to high levels of olefin and PNA saturation. FCC LCO also has a significant impact on reactor WABT. For example, an increase in %LCO from 20 to 40 corresponds to a required temperature increase from 50°F to 90°F, respectively. A summary of the process variable effects are summarized in Table 1.
Table 1. Process variable effects on catalyst life.
An Increase in the Following Conditions (e.g., WABT)
will Cause Product S, N or Aromatics to increase
or decrease, depending on the Specific Condition
| Specific Condition
(other conditions constant) |
Sulfur | Nitrogen | Aromatics | Catalyst life will ↑ or ↓, depending on specific condition |
| % Cracked Feeds | ↑ | ↑ | ↑ | ↓ |
| WABT | ↓ | ↓ | ↓ | ↓ |
| Feed Rate (LHSV) | ↑ | ↑ | ↑ | ↓ |
| Hydrogen Pressure | ↓ | ↓ | ↓ | ↑ |
| %h2s in Treat Gas | ↑ | ↑ | ↑ | ↓ |
As the feed end-point (EP) or final boiling (FBP) increases, this results in an increase in sulfur, nitrogen, % hard sulfur species, aromatics and PNAs. Directionally, this increases feed difficulty, which must be compensated for by increasing reactor temperature.
Causes of pressure drop
There are a variety of reasons for pressure drop in hydrotreaters, including feed quality, void fraction in the catalyst bed, liquid and vapor properties (i.e., density and viscosity) at reaction conditions, vapor and liquid superficial velocities, coke buildup and reactor internal problems.
In terms of feed quality affecting pressure drop, this is based on the amount and variety of particulates and contaminants in the feed. They can include iron sulfide, rust/scales, salts, coke fines, FCC catalyst fines and phosphorous. Poor equipment and line clean-up can further exacerbate problems from these particulates. Iron naphthenate formed from corrosion due naphthenic acids can cause iron to precipitate when heated and/or reacted with H2S. Other contaminant problems can include carbon and stainless steel sulfides from piping and furnace/exchanger tubes loosened during shutdown. Cracked stocks can form oxidation polymers when exposed to air. These deposit on the catalyst bed. In addition, diolefins/olefins can polymerize forming a gum on the catalyst bed.
Void fraction in the catalyst bed depends on catalyst size (diameter and length), catalyst shape and loading method (sock vs. dense method) and handling. The catalyst or support material breaks up during loading or operation. Careless handling and loading and rapid heating of “wet” catalyst can also increase catalyst bed void fraction.
Pressure drop problems also develop due to coke buildup result from excessive temperatures during operations or upsets that can cause coke lay down between (and on) catalyst pellets, as well as from heavy ends carry-over in feed.
Pressure drop from reactor internal problems may be traced back to torn support screens allowing catalyst into internals and plugging of support screens by corrosion products or fines. Plugging of the reactor outlet collector can be another reason for pressure drop.
Pressure drop buildup can be mitigated by feed filtration to remove particulates (at least 25 microns). It is important to ensure internals are assembled properly and remain clean during loading. Exposure of feedstocks (especially cracked stocks) to air in tankage should be minimized. Size-graded catalyst loading should be used for the top bed.
General Rules of Thumb Concerning Hydrotreater Reactors
- Hydrogen Consumption:
– Each °API increase results in approximately 100 scfb H2 consumption
– Each increase in Cetane number (1.0) leads to approximately 100 scfb H2 consumption in diesel
- Unless the feed is very high in sulfur, most H2 consumption comes from either PNA saturation or olefins
- Removal of contaminants (Si, Fe, Na, Ni, V, etc.) that deposits at 1.0 LHSV for one year will deposit on average across all the catalyst, about 1.0% of the catalyst weight. In addition, the top of the catalyst bed can easily collect 3-to-5 times the average for more reactive deposited materials and lead to plugging
- Most efficient systems exhibit SOR ΔPs of 0.5 to 1.0 psi/ft of catalyst bed in trickle flow. Good gas-liquid distribution is a must, and top bed size, void fraction and activity grading is a requirement.
Note: Refinery Operations extends its appreciation to the Grace Davison/ART organization for providing this beneficial information for the refining industry.
One response to “Identify Hydrotreating Process Variables”
Leave a Reply
You must be logged in to post a comment.







Key Operating Variables of Hydrocracking Unit;
https://thepetrosolutions.com/key-operating-variables-of-hydrocracking-unit/
Further, detail of WABT (Weighted Average Bed Temperature)
https://thepetrosolutions.com/weighted-average-bed-temperature-meaning-significance-and-calculation/